CD19-Targeted T-Cell Therapy Results in Safe, Rapid Reinduction of CR in Patients With Relapsed/Refractory B-Cell ALL
• Phase I trial: NCT01044069[1]
Summary of Key Conclusions
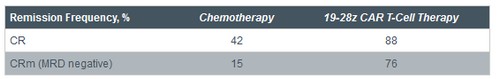
• In patients with relapsed/refractory B-cell acute lymphoblastic leukemia (ALL), CD19-targeted therapy with 19-28z chimeric antigen receptor (CAR) T cells produces rapid, high CR rates
• Similar efficacy in patients with minimal residual disease (MRD) and morphologic residual disease
• 70% of eligible patients transitioned to allogeneic stem cell transplantation (allo-SCT)
• Cytokine release syndrome (CRS) observed with 19-28z CAR T-cell treatment but can be effectively managed with steroids or tocilizumab
o Steroid treatment results in lymphotoxicity, leading to relapse
o No lymphotoxicity with tocilizumab
• Authors conclude reinduction rates and facilitation of allo-SCT justify further evaluation of 19-28z CAR T-cell therapy in phase II protocols
Background
• Up to 90% of adults with B-cell ALL achieve first CR with induction therapy
o Most relapse and become refractory to chemotherapy
o Allo-SCT standard of care for relapsed B-cell ALL
- Not all patients eligible
• New chemotherapy-free treatments needed for transplantation-ineligible relapsed patients
• 19-28z CAR T cells: novel CD19-targeted T-cell therapy
o T cells isolated from patients with relapsed/refractory B-cell ALL
- Genetically modified with CAR construct 19-28z CD19 binding domain fused to signaling domains of CD28 costimulatory receptor and zeta chain of CD3 receptor complex
- CD19: universal B-cell antigen expressed on normal and malignant B cells
o CD19 binding triggers cytotoxicity, cytokine release, and proliferation
• Role of CAR design in outcomes not yet fully understood
• Current study evaluated safety and efficacy of 19-28z CAR T-cell therapy in patients with relapsed/refractory B-cell ALL
Summary of Study Design
• Patients with B-cell ALL that is relapsed, refractory, MRD-positive, or in first CR
• Older than 18 years of age
• No exclusion for Philadelphia chromosome (Ph)–positive, extramedullary disease, central nervous system leukemia, and/or relapse following prior allo-SCT
• Treatment protocol for patients with relapsed B-cell ALL (n = 14)
o Leukapheresis
o Reinduction chemotherapy
o T-cell infusion (3 x 106 19-28z CAR T cells/kg)
o Monitoring for disease status and CAR T cells
- Polymerase chain reaction
- Flow cytometry
- Deep sequencing
• Treatment protocol for patients with B-cell ALL in first CR (n = 2)
o Leukapheresis followed by expectant monitoring
o Reinduction chemotherapy at relapse
o T-cell infusion, monitoring of CAR T cells and disease status as for relapsed patients
• Post–CAR T-cell treatment options
o Allo-SCT
o Different salvage therapy
o Monitoring, for patients not eligible for allo-SCT
Baseline Characteristics
• N = 16
• 86% of patients had residual disease after salvage reinduction chemotherapy prior to receiving CAR T-cell infusion

Main Findings
• 19-28z CAR T cells effective as salvage therapy
o Most patients induced into remission achieved complete molecular remission (CRm)
o Average time to CR: 24.5 days
• In patients with relapsed B-cell ALL and 60% to 70% bone marrow blasts after reinduction chemotherapy, rapid ablation of leukemia observed following CAR T-cell infusions[2]
o At 1-2 weeks post–CAR T-cell infusion
- Bone marrow aplastic
- Only CAR T cells present
o At 1-2 months post–CAR T-cell infusion
- Complete bone marrow restoration
- Functional and cellular recovery
• Adverse events observed
o Fevers
o Malaise
o Hypotension
o Hypoxia
o Neurologic changes
- Seizures
- Mental status changes
- Obtundation
• Toxicities correlated with pretreatment tumor burden
o Fevers
- Higher grade, longer duration in patients with pretreatment morphologic residual disease
- Low-grade fevers, if any, and shorter duration of fever with pretreatment MRD
o CRS also highest in patients with more residual disease (sIL2R?, TNF?, fractalkine, and GMCSF)[2]
- Management of CRS with steroids reduced fevers, but CAR T cells failed to expand in the blood and crashed soon after steroid treatment
- Management of CRS with tocilizumab reduced fevers without affecting CAR T-cell expansion
• Correlation with clinical outcomes
o All relapses (n = 3) occurred in patients treated with steroids
o No relapses in those treated with tocilizumab or managed conservatively
o 5-fold higher CAR T-cell expansion in bone marrow among patients with CRS and morphologic residual disease
• Allo-SCT post–19-28z CAR T-cell infusion
o 7 of 16 patients (44%) proceeded to allo-SCT to date, representing 70% of eligible patients
- 3 patients in CR medically ineligible for allo-SCT
- 1 patient pending evaluation with BMT service
- 2 patients declined transplant
o Post–allo-SCT follow-up: 2-24 months
o No post–allo-SCT relapses to date
Other Outcomes
• Pheresis products initiated with CD3 T cells ranging from 3.7% to 86%
• 15 of 16 patients achieved a CAR T-cell dose of 3 x 106 19-28z T cells/kg
• Average CAR T-cell transfer efficiency: 24% (range: 5% to 61%)
• Average CAR T-cell production time: 11 days
References
1. Davila ML, Riviere I, Wang X, et al. Safe and effective re-induction of complete remissions in adults with relapsed B-ALL using 19-28z CAR CD19-targeted t cell therapy. Program and abstracts of the 55th American Society of Hematology Annual Meeting and Exposition; December 7-10, 2013; New Orleans, Louisiana. Abstract 69.
2. Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38





